Semaglutide Rybelsus, Ozempic, NN9535, OG217SC, NNC 0113-0217 Maka iji nyocha naanị
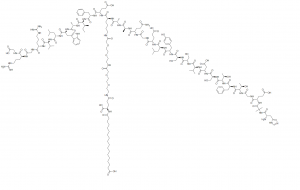
Semaglutide, rere n'okpuru akara ahaOzempic,WegovynaRybelsus, bụ iheọgwụ antidiabeticeji maka ọgwụgwọ nkeụdị ọrịa shuga 2na dị kaọgwụ mgbochi oke ibumaka ogologo ogenjikwa ibu, mepụtara site naNovo Nordiskn'afọ 2012. Semaglutide bụ aGLP-1 agonist anabata, nke pụtara na ọ na-eṅomi omume mmadụincretin glucagon-dị ka peptide-1(GLP-1), si otú ahụ na-abawanyeinsulinnzuzo na ịba ụbaọbara shugamkpofu na imeziwanyenjikwa glycemic.Mmetụta dị na ya gụnyere ọgbụgbọ, ọgbụgbọ, afọ ọsịsa, mgbu afọ, na afọ ntachi. Na Disemba 2017, akwadoro ụdị injectable aha ya bụ Ozempic.Na Septemba 2019, akwadoro ụdị nke enwere ike were n'ọnụ (Rybelsus), yana na June 2021, ntụtụ ọgwụ dị elu rere n'okpuru aha ika Wegovy maka njikwa ibu ogologo oge na ndị okenye ka ndị US kwadoro.Nchịkwa nri na ọgwụ(FDA).Na Jenụwarị nke afọ 2023, FDA nyere Novo Nordisk ikike ka ọ degharịa akara iji gosi na Rybelsus ọnụ nwere ike iji dị kaọgwụgwọ mbụmaka ndị okenye nwere ụdị ọrịa shuga 2 - nke pụtara na ndị na-aṅụbughị ọgwụ ọrịa shuga ọzọ. Na 2020, semaglutide bụ ọgwụ 129 nke a na-enyekarị na United States, yana ihe karịrị nde 4 nde ọgwụ. Ụdị okwu: Rybelsus, Ozempic, NN9535, OG217SC, Maka iji nyocha naanị.
Ọrụ ndu
| Nkọwa | Semaglutide (Rybelsus, Ozempic, NN9535, OG217SC, NNC 0113-0217), glucagon-dị ka peptide 1 (GLP-1) na-eme ogologo oge, bụ analog.GLP-1 nnabataagonist nwere ikike ịgwọ ụdị ọrịa shuga mellitus 2 (T2DM). |
| Ezubere iche | GLP-1 nnabata |
| In vitro | A na-ahọrọ Semaglutide dị ka onye kacha mma otu ugboro kwa izu.Semaglutide nwere nnọchi amino acid abụọ ma e jiri ya tụnyere mmadụ GLP-1 (Aib8, Arg34) ma wepụta ya na lysine 26. Mmekọrịta GLP-1R nke semaglutide (0.38 ± 0.06 nM) na-ebelata okpukpu atọ ma e jiri ya tụnyere liraglutide, ebe njikọ albumin. na-abawanye. |
| Na vivo | Ọkara ndụ plasma bụ 46.1 h na obere ezì na-esochi nchịkwa iv, yana semaglutide nwere MRT nke 63.6 h mgbe sc dosing to mini-pigs. |
Protocol (site na ntụaka)
| Nnyocha cell: | ● Ahịrị ekwentị:Mkpụrụ ndụ BHK ● Ntụkwasị obi:0.01 pm - 0.1 μM ● Oge nnabata:3 h ● Usoro:Mkpụrụ ndụ oyi kpọnwụrụ nke mkpụrụ ndụ BHK na-egosipụta ma hGLP-1R na CRE firefly luciferase (clone FCW467-12A/KZ10-1) na-agbaze, saa ya ugboro abụọ na PBS, ma kwụsịtụrụ na nchekwa nyocha.A na-atụgharị mkpụrụ ndụ n'ime efere 96 nke ọma na sel 5000 / nke ọma na olu nke 50 μL.Ngwakọta ndị a ga-anwale na-agbaze na ihe nchekwa nyocha yana aliquot 50 μL na-ebufe n'efere nke nwere sel iji rute mkpokọta nyocha ikpeazụ nke 1 × 10.-14- 1 × 10-7M. A na-etinye efere ahụ maka awa 3 na 5% CO2na 37 Celsius C.E kwere ka efere ahụ guzoro n'ime ụlọ okpomọkụ maka nkeji 15 tupu ịgbakwunye 100 μL nke steadylite gbakwunyere reagent.A na-ekpuchi efere ahụ iji chebe ya pụọ na ọkụ ma maa jijiji na ụlọ okpomọkụ maka 30 min.A na-agụ efere a na ngwa TopCount NXT. |
Solubility (25°C)
| In vitroOgbe: | DMSO | 3 mg/mL(0.73 mm) |
| Ethanol | Na-adịghị edozi | |
| Mmiri | Na-adịghị edozi |
Ozi kemịkalụ
| Ibu molekụla | 4113.58 | ||
| Usoro | C187H291N45O59 | ||
| CAS Mba. | 910463-68-2 | ||
| Nchekwa | Afọ 3 | -20°C | ntụ ntụ |
| afọ 2 | -80 Celsius | na ihe mgbaze | |
| Mbupu | Mbupu okpomọkụ ụlọ(Ọdịmma nchegbu efu: ngwaahịa ahụ dị mma na 37 ℃ maka opekata mpe otu izu.) | ||
Ozi nnwale ụlọ ọgwụ
| Nọmba NCT | Mbanye | Ntinye aka | Ọnọdụ | Onye nkwado/Ndị mmekọ | Ụbọchị mmalite | Usoro |
| NCT05537233 | Agaghị ewebata ọrụ | Ọgwụ: Semaglutide|Ọgwụ: Placebo | Ụdị ọrịa shuga 1|Obesara ibu | Mahadum nke Colorado Denver | Ntọala nyocha ọrịa shuga ụmụaka | Ọnwa Mbụ 1 2023 | Agba 2 |
| NCT04885634 | Agaghị ewebata ọrụ | Ọgwụ: Semaglutide Ngwaahịa Injectable | Ọgwụ: Placebo | Atrial Fibrillation | Ibu oke na oke ibu | Axel Brandes|Herlev na Gentofte Hospital|Hillerod Hospital Denmark|Svendborg Hospital|Ụlọ ọgwụ nke South West Jutland|Odense University Hospital | Ọktoba 2022 | Nkeji 3 |
| NCT05579977 | Na-ewe ndị ọrụ | Ọgwụ: PF-07081532|Ndị ọzọ: Placebo|Ọgwụ: Rybelsus | Ọrịa shuga mellitus | oke ibu | Pfizer | Ọktoba 27 2022 | Agba 2 |
| NCT05254314 | Na-ewe ndị ọrụ | Ọgwụ: Semaglutide Pen Injector 2.4mg kwa izu | Ndị ọzọ: Placebo | Asthma | Ụlọ Ọrụ Ahụike Mahadum Vanderbilt | Ụlọ Ọrụ Mba nke Allergy na Ọrịa na-efe efe (NIAID) | Septemba 7 2022 | Agba 2 |
| NCT05478252 | Na-ewe ndị ọrụ | Ọgwụ: Semaglutide J|Ọgwụ: Semaglutide B | Ọrịa shuga mellitus ụdị 2 | Novo Nordisk A/S | Ọgọst 3 2022 | Nkeji 3 |
(data sitere nahttps://clinicaltrials.gov, emelitere na 2022-11-29)